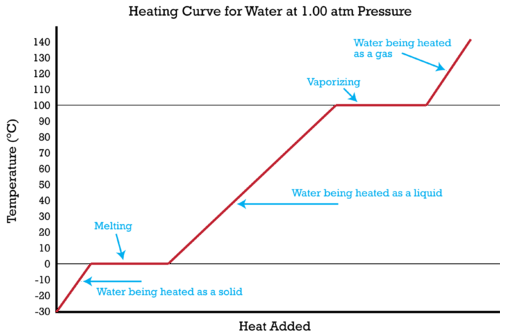
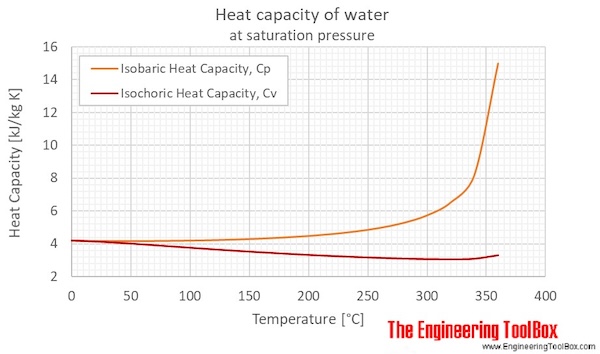
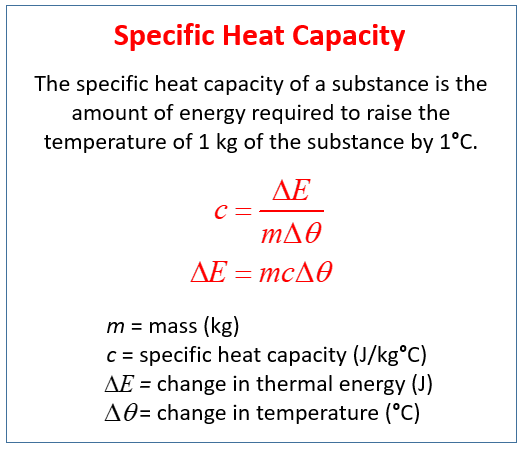
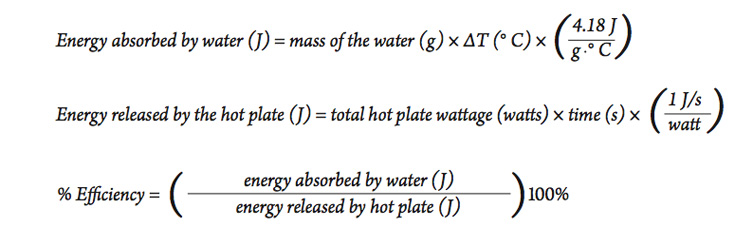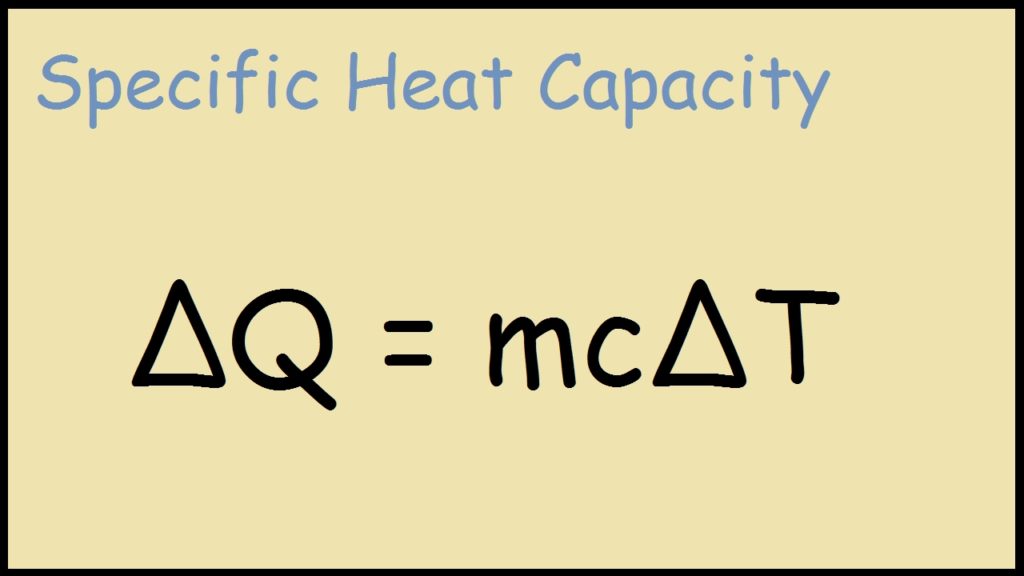How Much Thermal Energy Is Required To Heat Ice Into Steam - Heating Curve Chemistry Problems - YouTube

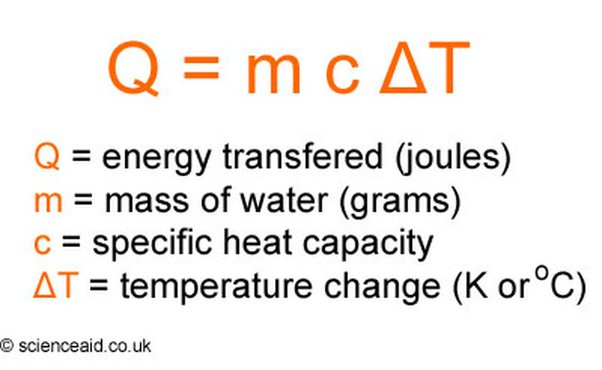
SOLVED: Use the heat equation to calculate the energy in calories used to heat 8.5g of water from 15°C to 36°C. Note: q = mCΔT C = 4.184 J/g 1 calorie = 4.184 J