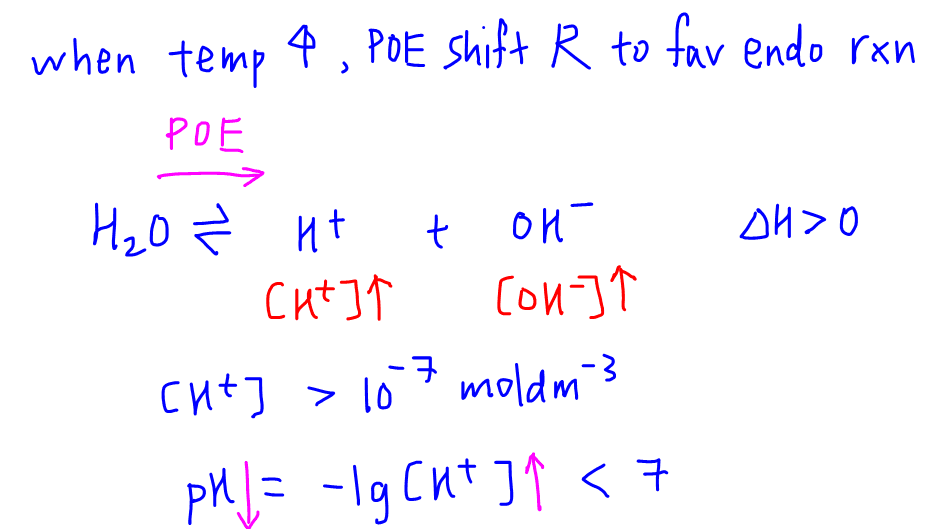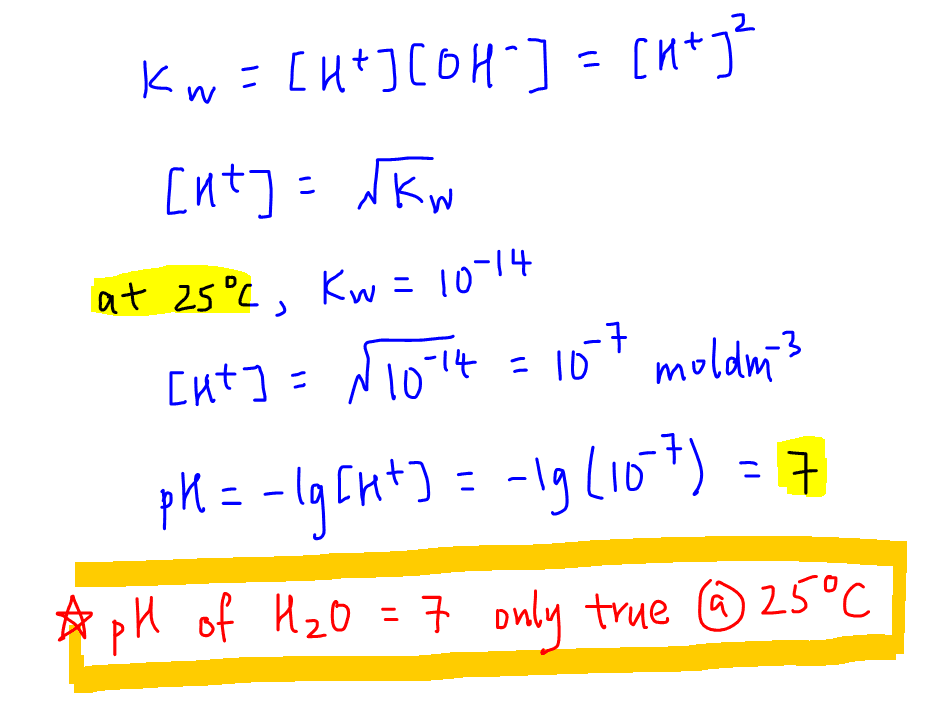9-19. Calculate the pH of water at 25°C and 75°C. The values for pKw at these temperatures are 13.99 and - brainly.com

The ionization constant water is 2.9x10-14 40°C. Calculate [H,O*), (OH), pH an pOH pure water 40°C. Ans : 1.703x10-7, 1.703x10-7, 6.7689, 6.7689

pH from Base concentration and Ionic Product of Water calculation Workthrough - A2 Chemistry - YouTube
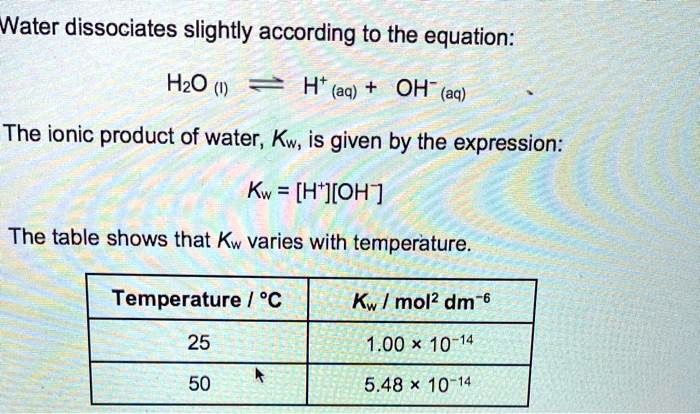
SOLVED: Calculate the pH of pure water at 50 degrees Celsius. Provide the answer to 2 decimal places and show your working. Water dissociates slightly according to the equation: H2O (l) ->

Unit 13 Acids and Bases. D. Finding the pH of Solutions Self- ionization of water – the simple dissociation of water H 2 O H + + OH - Concentration of. - ppt download


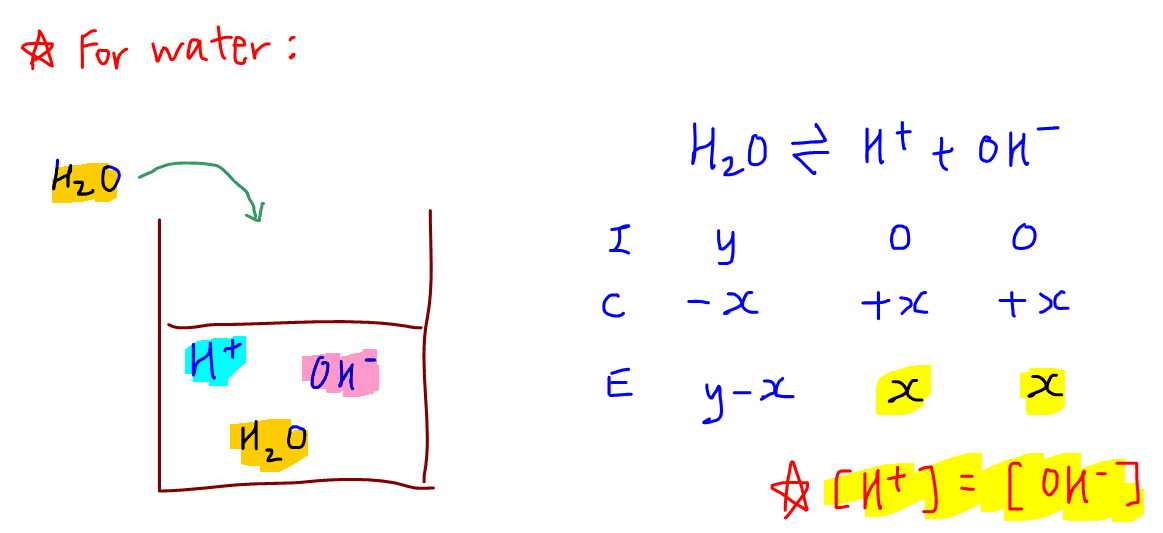

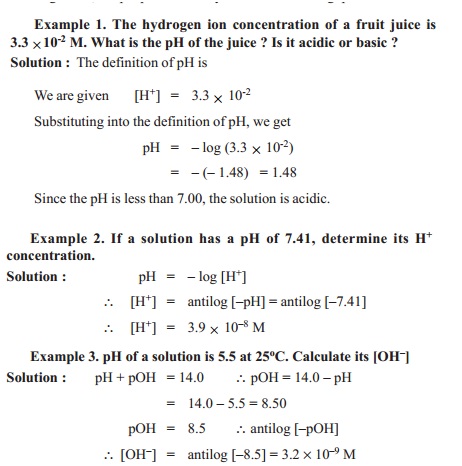



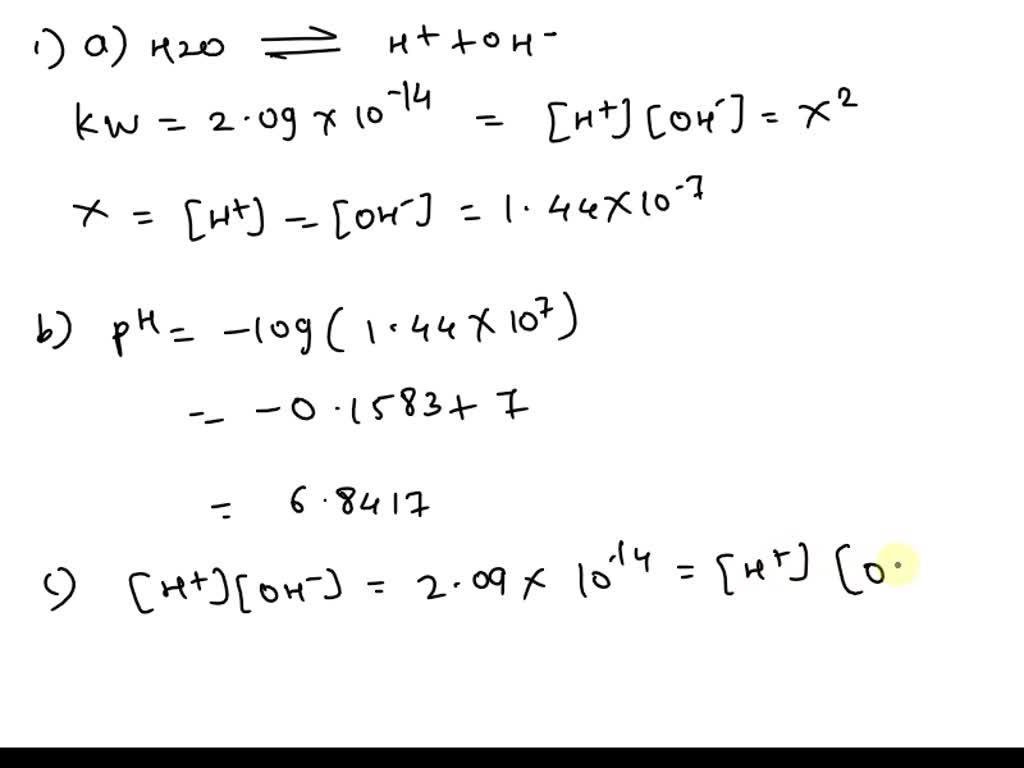


![Bengali] Calculate the pH of the following solution: 0.3g of NaOH d Bengali] Calculate the pH of the following solution: 0.3g of NaOH d](https://static.doubtnut.com/ss/web-overlay-thumb/3053784.webp)